Prediction of the Characteristics of Flame Retardant Coagent for Polyester Using Deep Learning Technology and Evaluation of the Retardancy
- Polymers
- Flame retardant
- Deep learning
- Prediction of the characteristics
Using deep learning technology, we searched for an alternative flame retardant coagent to antimony trioxide, which is a flame-retardant aid for polyester resins. Specifically, a deep learning model for physical property prediction was constructed based on the flame retardancy mechanism, and candidate materials were predicted and extracted. When the flame retardancy of the molding material using this candidate material was examined, it was found that it was equivalent to antimony oxide. In addition to flame retardancy, many physical properties of strength and fluidity are required for electronic components. However, since we studied an inorganic flame-retardant additive as an alternative to antimony trioxide, we assumed that the impact on other physical properties is considered to be minor, and here we will report only the flame retardancy.
1. Introduction
Polyester resins, such as PC, PBT, and PET, offer excellent electric insulation, heat resistance, and mechanical strength and are used for such mechanical devices as relays, switches, and connectors1). Flame retardancy is required for these resins to prevent fire. In order to satisfy this flame retardancy, halogen-based, especially a bromine-based flame retardant, is added to the resin. Furthermore, in polyester-based resins, such as PBT and PET, used for products requiring thin wall flame retardancy, antimony trioxide is used as a flame-retardant aid together with a bromine-based flame retardant in order to enhance flame retardancy2). Although the material with thin wall flame retardancy is essential for a part of electronic members, the situation is that development is not actively performed because of the effect on investments in raw material manufacturers.
On the other hand, antimony trioxide used as a flame-retardant aid is published as a candidate in the RoHS Directive, and the environmental risk is a concern3). In addition, the geopolitical risk is considered to be high because mining the place of antimony is very unevenly distributed4). With such a background, although each of the raw material manufacturers has studied a variety of materials as alternative materials for flame retardancy, no flame-retardant aid instead of antimony trioxide has been discovered since 1920. Therefore, it is supposed to be difficult to find alternatives for antimony trioxide in the conventional search range. Although it is necessary to enlarge the search region to discover new alternative materials, the studies of enormous combinations are difficult by conventional approaches mainly relying on experiments5).
In recent years, materials informatics (MI) has attracted attention. MI is a new approach method for performing material development utilizing information science, and a compound that was not solved by the conventional approach was discovered, and the development period was shortened successfully6). Among them, the Massachusetts Institute of Technology and Samsung Electronics found new safe and long-life solid electrolytes for lithium batteries using MI in a short period7). Like this, the utilization of MI is effective for studies that require a long time to discover highly functional raw material, and it is a technology that enables significantly shortening the lead time for technical development.
Notwithstanding that this MI is the means for the effective discovery of new compounds, utilization has often been limited to basic research, such as utilization of super computers only by universities and research facilities dedicated to raw material development and enterprises with basic research institutes. However, the environment enabling utilization of MI has improved by forming an alliance with venture enterprises having the business of MI of which the number increased in recent years, in addition to the significant performance improvements of personal computers.
Therefore, we have challenged the search for flame-retardant aid with a polyester base in the new approach method utilizing MI by forming an alliance with an enterprise possessing MI. We built a deep learning model that predicts the bromination energy of flame-retardant aid reacting with a flame retardant and the boiling point showing the ease of volatilization of the bromized flame-retardant aid from the mechanism of flame retardancy, and we predicted and extracted candidate agents. We report on the material that we discovered had the characteristics equivalent to antimony trioxide from them.
Furthermore, although a non-halogen-based flame retardant not using a bromine-based flame retardant is the general type to deal with the RoHS Regulation in the world, the approach to non-halogen-based flame retardants was excluded. The reason is that we judged that the non-halogen-based flame retardant could not satisfy the coexistence of thin wall flame retardancy and strength characteristic that we require because the flame retardant effect of non-halogen-based flame retardants is low in comparison with that of halogen-based flame retardants, and the increase of blending quantity is supposed to lead to the reduction in the property of resin.
2. Approach method
2.1 Understanding the flame retardancy mechanism and extraction of important parameters
First, we explain the important parameters regarding the flame retardancy mechanism of flame retardants and flame-retardant aid. Generally, it is well known that the flame retardancy of resin expresses a high effect from the synergistic effect of the combination of the flame retardant and the flame-retardant aid8). Halogen-based flame retardants and the antimony trioxide mentioned above are listed as representative. This is caused by the chain reaction stop action (radical trap effect) of the thermal decomposition of the reaction product by the halogen compound and antimony and the air shutoff effect in the gas phase by vaporization of halogenated antimony1).
We now consider the mechanism in detail. Antimony trioxide (Sb2O3) that is a flame-retardant aid is bromized by HBr produced by the decomposition of the bromine-based flame retardant. This vaporized bromized antimony (Sb2Br3) hinders the combustion reaction in an oxidation reaction system. We study the combustion mechanism of resin containing the various bromine-based flame retardants with added Sb2O3 as the flame-retardant aid. The produced SbBr3 reacts with the hydrogen atom and is converted to HBr, SbBr, SbBR2, and Sb. In particular, Sb reacts with oxygen atoms, water molecules, and hydroxyl group radicals, then SbO and SbOH are produced and the cutoff of the chain reaction of combustion hinders the reaction8).
We considered that the bromide is immediately diffused to make oxidation easy after flame-retardant aid is easily bromized and its bromide is further instantly vaporized, and this ease of oxidation is important from the above-mentioned reaction mechanism. That is to say, we considered that two viewpoints are important characteristics in this flame-retardant aid from the above-mentioned content.
- (i)
- Flame-retardant aid by which bromide is easily produced
- (ii)
- Produced bromide of (i) is easily vaporized
Item (i) shows that the HBr generated from the flame retardant easily reacts with the flame-retardant aid, that is to say, the smaller the Gibbs free energy change (bromination energy) before and after the reaction in the bromination reaction of the flame-retardant aid, the easier the aid is bromized. In addition, the reason for the easy vaporization of (ii) is presumably that the boiling point of the substance itself is low.
2.2 Method for estimating physical properties
Concerning bromination energy and the boiling point that are important characteristics of the flame-retardant aid extracted in 2.1, it is preferable to extract candidate agents where the bromination energy is low and where the boiling point is low. However, these characteristics are found only in a part of the literature, and the material search has been limited. Therefore, we decided to calculate the bromination energy and boiling point based on the thermodynamic calculation and deep learning model together with the data in books, papers, and websites to supplement the data.
(1) Calculation of bromination energy
Thermodynamic calculation was performed using the simplified reaction formula shown in Fig. 1 based on the report of Shibata et al.8) The specific explanation of taking metal oxide as an example is that we calculated the difference of the Gibbs free energy before and after the reaction using the Gibbs free energy9) of the metal oxide and the metal bromide and calculated the bromination energy (ΔG). This time we decided to simply assume the bromination reaction by the oxide and HBr to be shown by the following formula and to calculate the bromination energy based on this formula.
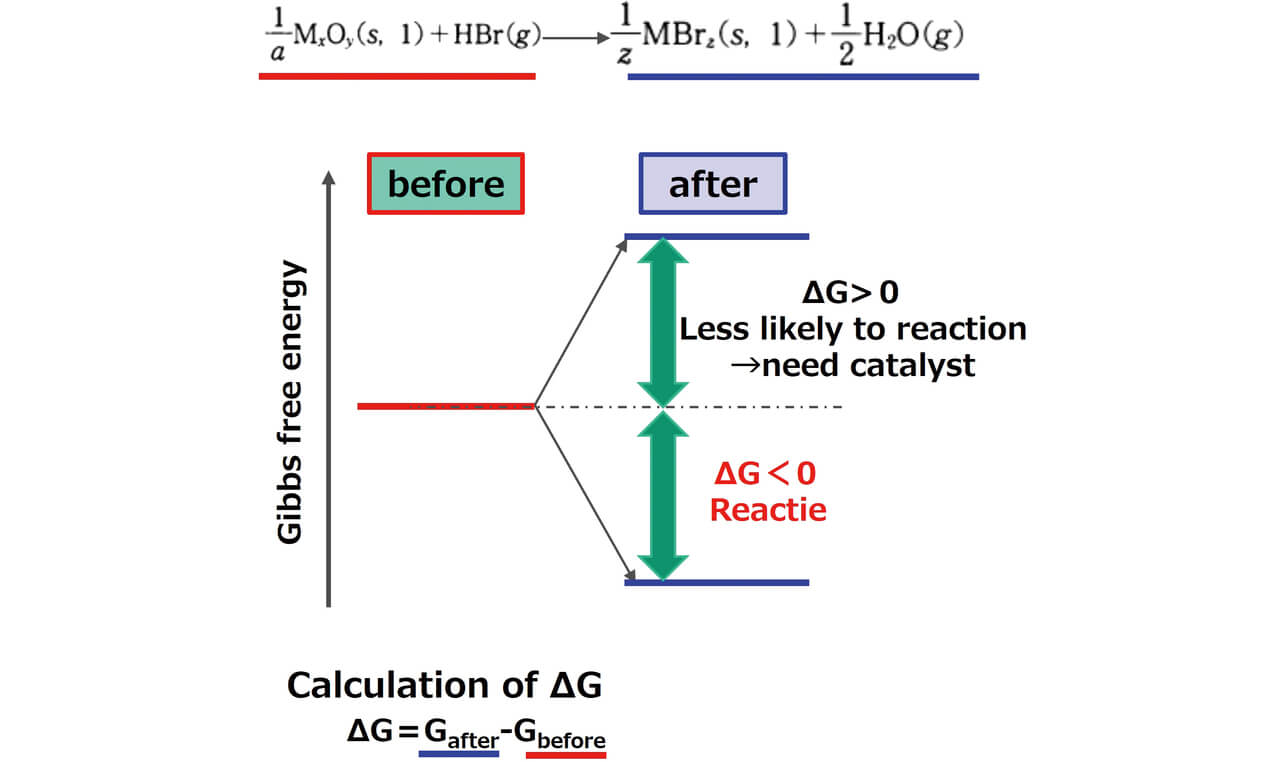
We calculated the bromination energy change by a similar approach for compounds, such as other metal hydroxides. In addition, we decided to adopt the value of the bromination energy near the temperature of approx. 700 K, which is the decomposition temperature of general molding materials.
(2) Calculation of boiling point of bromide
We adopted the temperature in which the solid phase and the gas phase of metal bromide become the same using the Gibbs free energy of the metal bromide as the boiling point of the bromide.
(3) Building deep learning model
Deep learning is a technique of machine learning that derives the result (output) corresponding to input data. This time we used the models of two logical structures (Graph Isomorphism Network (GIN) and Attentive Fingerprints (AttentiveFP)) for deep learning. We used SMILES, which stringizes the chemical structure of a molecule using alphanumeric characters for input. In the case of bromination energy, we also used a ŌĆ£FlagŌĆØ encoded by two bits of the physical condition of the compound.
We predicted the Gibbs free energy and boiling point by calculation of the deep learning model and estimated the properties related to compounds not contained in the value of the literature and the data of thermodynamic calculation. Furthermore, we verified the accuracy of this deep learning model based on the correlation between the data of actually measured values shown in the next Section and the numerical values calculated for the deep learning model. In order to improve the accuracy of deep learning, we executed deep learning, including the data of the Gibbs free energy and its boiling point regarding the chemical reaction of halogen-based material together with the values of the above-mentioned Gibbs bromination energy and boiling point.
2.3 Retardancy test
Test pieces were prepared by mixing two materials consisting of the antimony trioxide or the flame-retardant aid obtained from the verification of the deep learning model and the bromized flame retardant in the polyester-based resin and performed injection molding. We attached the test piece (125┬▒5├Ś13┬▒0.5├Śt mm: t’╝Ø0.4 mm) to a clamp vertically, performed contact with a 20 mm flame for 10 seconds twice, and judged flame retardancy based on the combustion behavior (conforming to UL94). V-0 shows the highest flame retardancy according to UL94, and V-1 and V-2 are nonconforming.
3. Results and discussion
3.1 Reliability of data of deep learning model
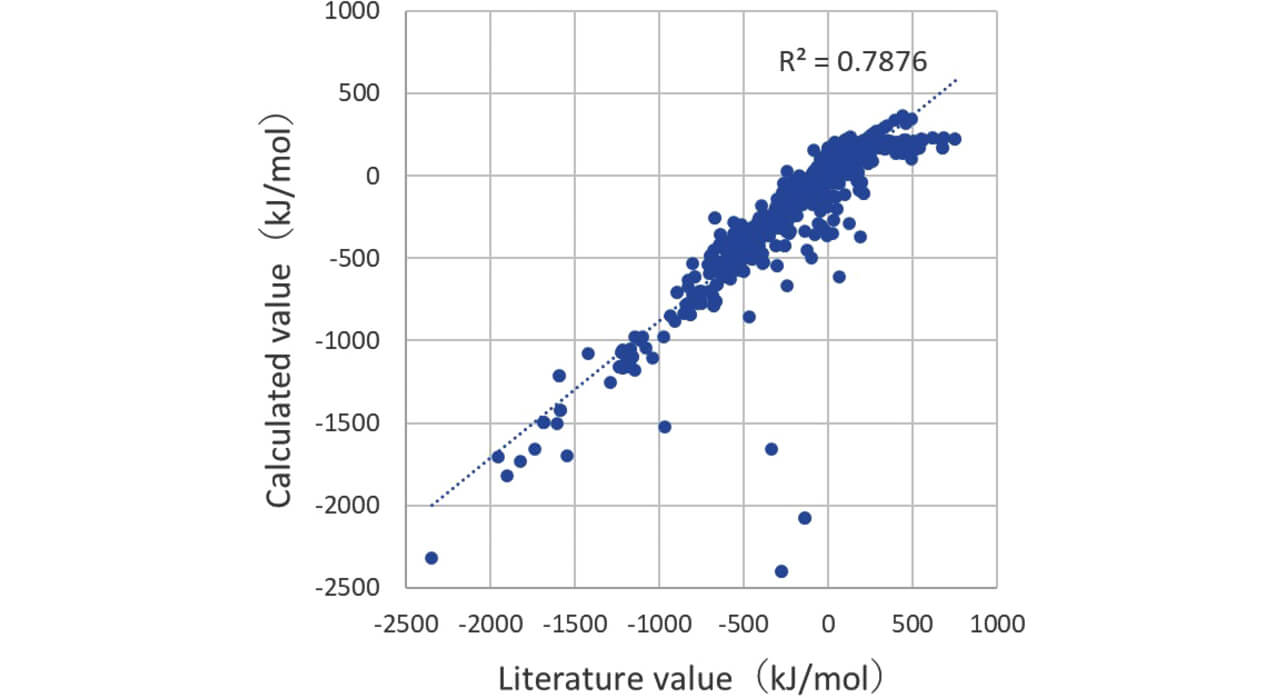
The data of the plotted calculated values and literature values of bromination energy in order to verify the reliability of deep learning model are shown (Fig. 2). Correlation coefficient R2 between the calculated value and the literature value by the deep learning model was 0.79, and a strong correlation was recognized. Furthermore, the data largely running off from the straight line showing the correlation between the calculated value and the literature value were generated by the deviation of the learnt data quality (coverage range) and the small amount of data. It is considered that the addition of learnt data helps to obtain data with a stronger correlation.
3.2 Calculated values of boiling point
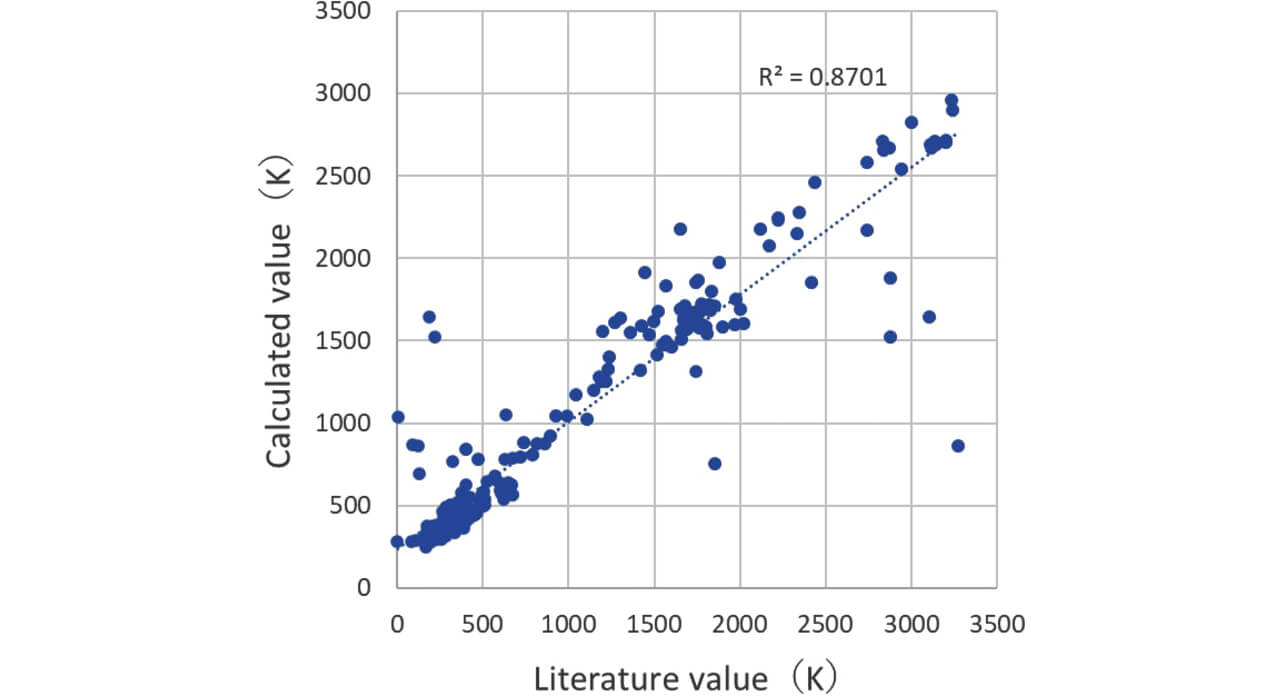
The data of the plotted calculated values and literature values of the boiling point are shown in Fig. 3. Also, in the calculated values of the boiling point, the correlation coefficient R2 was 0.87, and a strong correlation was recognized.
3.3 Extraction of candidate agent
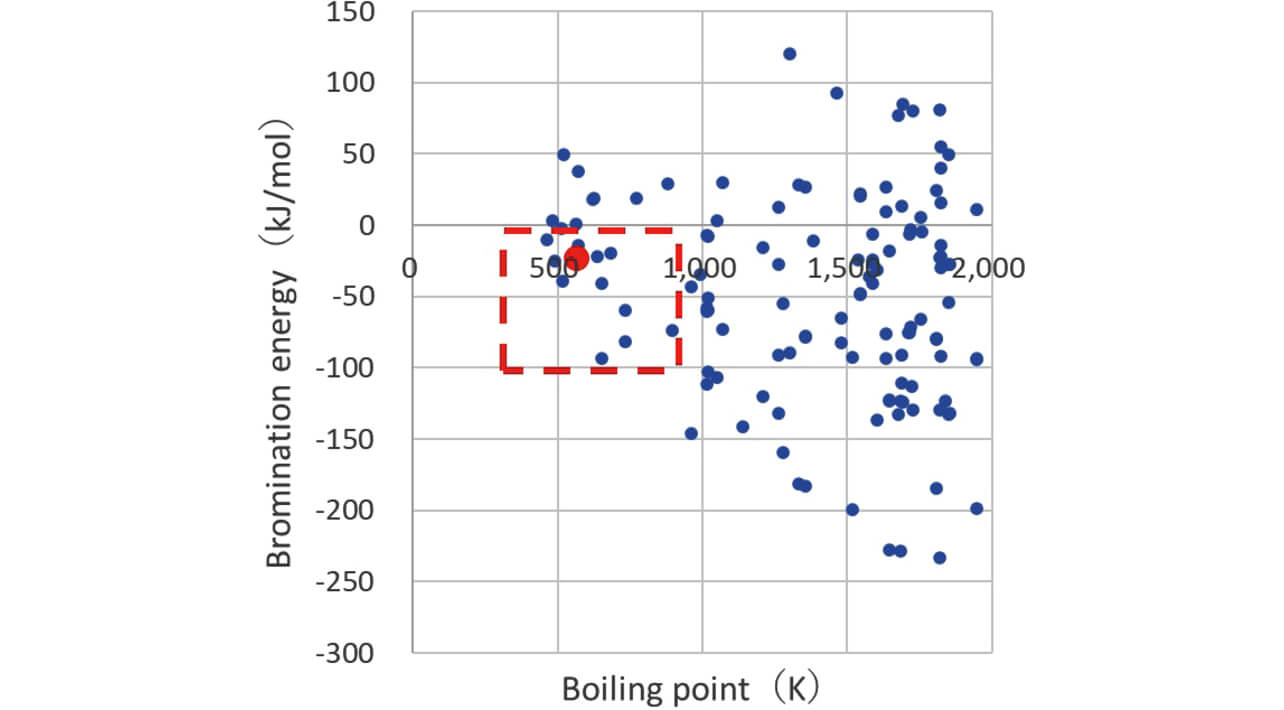
The X-axis and the Y-axis represent the boiling point and bromination energy obtained by MI, respectively, and the data were plotted as shown in Fig. 4. The graph shows a fan-like shape widening from the upper right of the graph. The elements (i) where the boiling point was low (equal to or less than general decomposition temperature of resin 700 K’╝ŗ100 K) and (ii) where the bromination energy was low (zero or less at which reaction easily progresses) that were set as the required characteristics of the candidate agent in 2.1 were extracted (surrounded by red broken line frame). Antimony trioxide (red dot) was also contained within this region. We excluded the compounds that violated the Industrial Safety and Health Law and the Poisonous and Deleterious Substances Control Act from the viewpoint of the environment, the substance without novelty as a flame retardant, and the materials that are seldom available from these, and consequently extracted two types of compounds as candidate agents.
3.4 Flame-retardancy test
The above-mentioned two types of compounds were extracted, and the flame-retardancy test was conducted together with antimony trioxide. Furthermore, proper amounts of flame retardant and flame-retardant aid were added in the flame-retardancy test, which was conducted based on the UL test. It is known from past tests that the combination of antimony trioxide and bromine-based flame retardant helps to secure high flame retardancy (V-0). This time, in order to verify the significant difference in flame retardancy between the antimony trioxide and the flame-retardant aid of the candidate agent, we adjusted the compounding ratio of flame retardants so that flame retardancy was V-2 in the combination of the bromine-based flame retardant and antimony trioxide and produced molding material experimentally. In addition, we adjusted the amount of the candidate agent added so that the relationship of the mol ratio of antimony trioxide to polyester was constant from the viewpoint of a chemical reaction. As shown in Table 1, the result is that the flame retardancy of compound A is lower than that of antimony trioxide in the region of small thickness. However, flame retardancy was able to be secured by securing thickness. On the other hand, compound B has flame retardancy equivalent to that of antimony trioxide even in the region of small thickness. Since our electronic components include molding requiring thin-wall flame retardancy, we judged that compound B was a flame-retardant aid suitable for the antimony trioxide alternative.
| Type of flame-retardant aid | Thickness of molding material (mm) | ||
|---|---|---|---|
| 0.4 | 0.8 | 1.6 | |
| Antimony trioxide | V-2 | V-2 | V-2 |
| Compound A | Nonconforming | V-2 | V-2 |
| Compound B | V-2 | V-2 | V-2 |
4. Conclusions
Since antimony trioxide of the polyester-based resin used for electronic members, such as the mechanism device, is a candidate substance of the RoHS Regulation, we tackled the search for alternative material this time.
Although we do not accumulate and possess much material property data like material manufacturers, we found the possibility that we could promote the search for new compounds in the niche region required in-house by making full use of the mechanism, theoretical formula the and the literature. We were able to search for a flame-retardant aid as an antimony trioxide alternative to the flame-retardant aid of a polyester-based resin this time from the prediction of the characteristics necessary for flame-retardancy characteristics utilizing deep learning technology. We built a deep learning model for physical property prediction based on the flame-retardancy mechanism and predicted and extracted candidate agents. We found that the characteristics of the flame-retardant aid of this candidate agent were equivalent to that of antimony trioxide.
Since we studied inorganic compounds as an alternative for antimony trioxide as a flame-retardant aid of bromine-based flame retardants this time, the risk of strength reduction is low, and we did not perform a strength evaluation. Hereafter, we promote the evaluation of material characteristics, including strength being required as characteristics other than flame retardancy. In addition, we promote the verification of the mass productivity of polyester resin containing the candidate agent together with a search for new compounds while watching the tendency of the RoHS Regulation.
Acknowledgments
We sincerely thank Representative Shinya Yuki of Elix, Inc., who formed an alliance with us, and members who cooperated during these studies.
References
- 1’╝ē
- M. Okoshi, ŌĆ£Flame-proofing of plastics,ŌĆØ (in Japanese), Molding Process., vol. 29, no. 12, pp. 449-455, 2017.
- 2’╝ē
- H. Nishizawa, ŌĆ£Bromine-based flame retardant,ŌĆØ (in Japanese), J. Soc. Rubber Sci. Technol., Jpn., vol. 92, no. 6, pp. 211-217, 2019.
- 3’╝ē
- RoHS Directive ŌĆ£DIRECTIVE 2011/65/EU,ŌĆØ Article 6 (Review of limited substances)
- 4’╝ē
- S. Ishihara and T. Ohono, ŌĆ£Present situation concerning antimony mineral resource in the world,ŌĆØ (in Japanese), Resour. Geol., vol. 62, no. 1, pp. 91-97, 2012.
- 5’╝ē
- H. Nishizawa, Basic Understanding of Flame-proofing Technology. (in Japanese), Kogyo Chosakai Publishing, 2004.
- 6’╝ē
- T. Chikyow, ŌĆ£Basic understanding of flame-proofing technology,ŌĆØ (in Japanese), J. Jpn. Soc. Info. Knowl., vol. 4, no. 27, pp. 207-211, 2017.
- 7’╝ē
- Y. Wang, W. D. Richards, S. P. Ong, L. J. Miara, J. C. Kim, Y. Mo, and G. Ceder, ŌĆ£Design Principles for solid-state lithium superionic conductors,ŌĆØ Nat. Mater., vol. 14, pp. 1026-1031, 2015.
- 8’╝ē
- E. Shibata, M. Grabda, and T. Nakamura, ŌĆ£Thermodynamic study concerning bromination reaction of inorganic compounds,ŌĆØ (in Japanese), J. Jpn. Soc. Waste Manage. Experts, vol. 17, no. 6, pp. 361-371, 2006.
- 9’╝ē
- I. Barin, Thermochemical Data of Pure Substances, 3rd ed., Wiley, 1995.
The names of products in the text may be trademarks of each company.