Notice regarding Capital and Business Partnerships with AliveCor
March 17, 2017
OMRON Healthcare Co., Ltd. (Head Office: Muko, Kyoto, Japan, President & CEO: Isao Ogino, hereinafter referred to as "OMRON Healthcare") announced today an agreement to form capital and business partnerships with AliveCor, Inc. (Headquarters: Mountain View, California, U.S., CEO: Vic Gundotra, hereinafter referred to as "AliveCor"), which provides diagnosis and treatment support services for atrial fibrillation (AF)*1 in the U.S. and other territories.
With this agreement, OMRON Corporation will contribute capital in the amount of USD25 million (approx. JPY2.87 billion) to AliveCor. Meanwhile, OMRON Healthcare will forge a business partnership with its new American partner in which the two companies will have mutual access to the premium products and services that they offer in the cardiovascular disease (CVD) field, thereby increasing its ongoing efforts to "zero cerebral and cardiovascular events."
Incidence of cerebrovascular and cardiovascular diseases, such as cerebral infarction and myocardial infarction caused by hypertension, can lead directly to death, or leave patients bedridden or with speech disabilities, which can result in a considerable decrease in the quality of life (QOL) of the patients, as well as their families. Even now, the number of hypertensive patients in the world is estimated at 1 billion, and 17.5 million people are said to suffer from cerebrovascular or cardiovascular events caused by high blood pressure.
As a leading company in the CVD business with its main product being blood pressure monitors, OMRON Healthcare has set its business vision to "zero cerebral and cardiovascular events," and is forging ahead with the development of technologies, products, and services to realize this vision.
AliveCor is a renowned provider of advanced support services for diagnosis and treatment of AF through the supply of mobile and wearable cardiographs and a remote patient monitoring platform used by doctors.
Since debuting the world's first mobile cardiograph unit (FDA-cleared) in 2012, AliveCor has delivered a variety of premium products and services. Starting in March 2017, the American company will launch its "Kardia Pro" remote monitoring platform, which allows specialists to monitor patients' electrocardiography (EKG) data remotely and perform diagnosis and treatment. With products and services ranging from mobile and wearable EKG equipment to a unique algorithm for diagnosis of AF and a remote monitoring platform directly connecting patients with 30,000 cardiologists across the U.S., AliveCor is increasingly committed to strengthening its efforts to provide doctors with a seamless system that is geared toward reducing the incidence of cardiovascular events.
It has been determined that integrating EKG data, which are closely linked to blood pressure data, to achieve simultaneous management and analysis, will make it possible to estimate patients' risk of developing cerebrovascular and cardiovascular events with even greater accuracy. This determination led to the decision to contribute capital to and form a business partnership with AliveCor, a leading player in the CVD field with a highly reputed mobile cardiograph product. Details of the business partnership are as follows.
Distribution of AliveCor's mobile EKG monitoring units through OMRON Healthcare's sales networks
OMRON Healthcare will distribute AliveCor's mobile EKG monitoring unit Kardia Mobile through its sales networks in Europe, as well as in the U.S., in a bid to make AliveCor's premium AF monitoring and diagnosis support available in an extended range of territories.- Incorporation of OMRON Healthcare's remote blood pressure monitoring service into "Kardia Pro"
OMRON Healthcare will incorporate its remote blood pressure monitoring service "Medical LINK*2," which is currently being offered in Japan, into the "Kardia Pro" platform service for remote monitoring of EKG data and diagnosis/treatment that AliveCor will launch this March. This will make it possible to manage EKG and blood pressure data simultaneously on a single platform. - Joint development of a new algorithm and services for the prevention of CVDs
Integrated analysis of EKG and blood pressure data will increase accuracy in assessing the risk of CVDs. Under this new partnership, the two companies will pool their knowhow to embark upon development of an algorithm that can achieve integrated analysis of EKG and blood pressure data and calculation of risk scores, as well as services that incorporate such an algorithm. - Joint development of a device
The two companies will jointly develop a device that measures EKG while estimating blood pressure, which will make it possible for users to gather data necessary for calculating the risk of CVDs without any additional effort.
*1 Atrial fibrillation occurs when normal electrical impulses are disrupted by irregular discharges, which then disrupt the movement of the atria.
*2 Developed under the supervision of hypertension specialists, Medical LINK is OMRON Healthcare's unique blood pressure analysis service for medical institutions.
Blood pressure data collected by patients at home are sent automatically to a server and then used to monitor trends in such data and calculate morning and evening averages in accordance with the guidelines on hypertension treatment.
Medical LINK® supports optimization of physicians' medical services by displaying on their computers various graphs and detailed analyses of characteristics that would normally be difficult to glean only from patients' blood pressure notebooks.
About AliveCor, Inc.
AliveCor, Inc. is pioneering the creation of FDA-cleared 'machine learning' techniques to enable proactive heart care and is recognized around the world for transforming cardiac care. The FDA-cleared Kardia Mobile is the most clinically validated mobile ECG solution on the market and is recommended by leading cardiologists and used by people worldwide for accurate ECG recordings. This simple-to-use mobile device and app-based service provides instant analysis for detecting atrial fibrillation (AF) and normal sinus rhythm in an ECG. Kardia Pro is the first artificial intelligence-enabled platform for doctors to monitor patients for the early detection of atrial fibrillation, the the most common cardiac arrhythmia that leads to a five times greater risk of stroke. AliveCor was recognized by Fast Company as one of 2017's most innovative companies in health (#3). AliveCor is a privately-held company headquartered in Mountain View, Calif. For more information, please visit alivecor.com.
Kardia Mobile and the Kardia app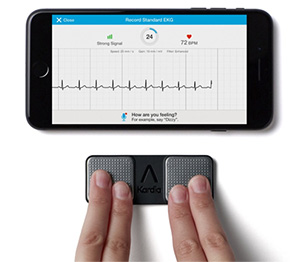
EKG measuring unit with Apple Watch support Kardia Band
Kardia Pro patient file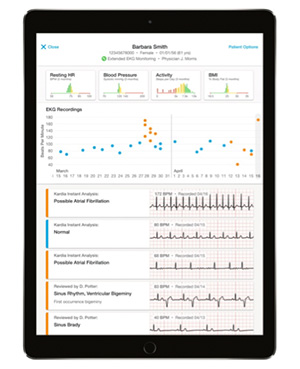
About OMRON Healthcare
Committed to improving people's lives, OMRON Healthcare provides clinically proven, innovative medical equipment for health monitoring and therapy. Our product portfolio includes blood pressure monitors, nebulizer, electronic thermometers, blood glucose monitors and activity counters as well as body composition monitors and professional medical devices. For many decades, OMRON's devices have helped people to prevent, treat and manage lifestyle diseases both at home and in clinical practice in more than 100 countries in the world. OMRON Healthcare Group is headquartered in Muko City, Kyoto Prefecture, Japan. Its President is Isao Ogino.
OMRON Healthcare website: http://www.healthcare.omron.co.jp/english/






