October 05, 2020
Story Behind the Rise of the World's First FDA-Approved Wearable Blood Pressure Monitor
- Two Engineers Who Tackled the Long-Standing Challenge of Downsizing Blood Pressure Monitors -
Essential at any medical consultation, blood pressure monitors play an indispensable role in the early detection of abnormal blood pressure. In Japan, it is estimated that 43 million*1 persons are hypertensive, about one-third of the entire population. One of the issues that need to be addressed regarding hypertension is that, because it is mostly asymptomatic, not many hypertensive patients proactively seek treatment. If left untreated, however, it can cause serious diseases, such as myocardial infarctions and strokes. Applying innovative technologies to solve social issues, OMRON has developed wearable blood pressure monitors that are significantly smaller than conventional models in a bid to produce devices designed to achieve zero cerebrovascular and cardiovascular events caused by high blood pressure. Approved by the authorities in the U.S. and Japan, this milestone device assists medical professionals in the diagnosis and treatment of hypertension.
*1 Estimated by OMRON from the 2016 National Health and Nutrition Survey data, Ministry of Health, Labour and Welfare of Japan
The Next Steps to Take after a Lengthy Period Convincing the Public of the Merits of Home-use Blood Pressure Monitors
OMRON released its own home-use blood pressure monitors in 1973. Since then, OMRON has been working closely with medical professionals to popularize home-use blood pressure monitors, while at the same time enhancing the accuracy and usability of the device. Their efforts paid off in 2003 when the Japanese Society of Hypertension (JSH) specified the method and standard for measuring blood pressure at home in the JSH Guidelines for Self-Monitoring of Blood Pressure at Home. They then took another step forward to stipulate, "When blood pressure taken at a doctor's office differs from that taken at home, the latter should take precedence" in their Guidelines for the Management of Hypertension 2014. The fact that blood pressure monitoring at home was validated as a method of hypertension care was a huge stride in preventing the onset of cerebrovascular and cardiovascular disorders triggered by hypertension.
As clinical research on home blood pressure monitoring subsequently grew, both in depth and variety, it became known that blood pressure varies throughout the day and that major fluctuations in blood pressure during the day raises the risk of developing cerebrovascular and cardiovascular disorders. The spread of home-use blood pressure monitors increased the frequency of measurement, but they were still too bulky to be carried around. Thus began OMRON's new challenge - keeping track of daytime fluctuations of blood pressure to achieve its goal of Going for Zero (refers to preventing the onset of serious events attributable to hypertension).
Continuous Wearing - In Pursuit of Preventing the Sudden Onset of Cerebrovascular/Cardiovascular Disorders
OMRON's brand new wearable blood pressure monitor is the brainchild of Nishioka Takanori and Kubo Takeshi, both talented engineers who are responsible for advanced product development at OMRON HEALTHCARE Co., Ltd.
Someone close to Nishioka once had a cerebral infarction, and Nishioka witnessed the dramatic changes that subsequently took place in the life and financial condition of that person and his family. Kubo, on the other hand, had a family member and a friend who collapsed from brain/heart diseases, so the two engineers shared a strong wish to save as many persons as possible from the dangers of hypertension and to raise awareness about blood pressure among younger generations.
They began asking themselves exactly what kind of a product they should aim for that could make Going for Zero a reality. After much thought, they concluded that it had to be something that "one could wear all the time like a wristwatch so they could take measurements anytime, anywhere." If continuous monitoring could be realized, it should be possible for each individual to measure the fluctuations in their blood pressure as they dealt with various psychological states and circumstantial stressors. If people knew how much risk they were running of developing cerebrovascular and cardiovascular events, they might well begin changing their lifestyles for the sake of prevention or reach out for medical help and treatment, which in theory should help to achieve Going for Zero.
Easier said than done - Soon they realized the daunting challenges - for continuous monitoring to be realized, they had to drastically downsize every single component, not to mention they would have to maintain the accuracy of measurement and the measurement principle while doing so.
Triple Cuff Configuration Breaking through the Restrictions of the Oscillometric Method *2
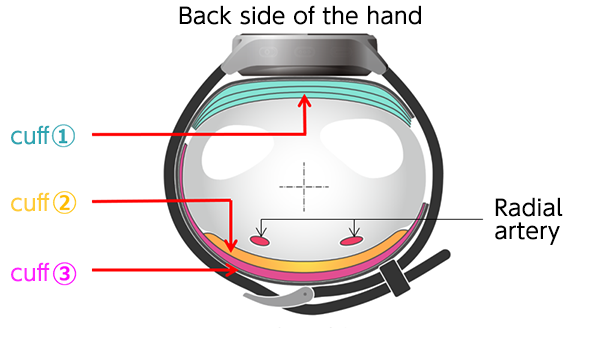
Cross-sectional View of the Cuff Structure
The first wall they came up against in their pursuit of downsizing components was the existence of cuffs (airbags inside the inflating portion of blood pressure monitors), which cannot be done without when measuring blood pressure. In order to correctly measure blood pressure, arteries need to be compressed by feeding air into the cuffs. To this end, cuffs had to be 52 mm wide for the prevailing models at the time. For the monitor to be worn continuously and comfortably, however, the width had to be less than half of the standard size.
With the popular models of wrist blood pressure monitors at the time, the single cuff played two different roles: it would slowly compress arteries by pressuring the wrist while detecting the changes in pressure inside the cuff and comparing them to the changes resulting from the subtle pressure oscillation that occurs in response to the pulsation of blood pressure in the arteries. This being the case, if the cuff were made too narrow, it would need higher pressure to compress the arteries, which would widen the gap with the pressure being placed on the arteries. It would also make it difficult to stably detect the pulse wave curve, which would result in poor accuracy of measurements. Furthermore, in light of restrictions of parts and materials used and production costs, no one questioned the long-standing notion that the cuff must be 52 mm or wider.
But all this did not deter Nishioka, and only served to ignite his passion as an engineer to develop the world's smallest blood pressure monitor. Kubo felt that they should discard all existing principles and methodologies, and instead start from scratch without being restricted by the deep ruts formed by the existing engineering knowledge. Even in the face of difficulty, they believed that they could achieve a breakthrough in this exciting challenge if they joined hands with each other to accomplish the mission of devising a new generation of blood pressure monitors whose performance would change patients' lives for the better.
Between 2016 and 2018, they made and tested countless prototypes. Day in, day out, they spent time analyzing the test results, tirelessly repeating their process each proceeding day. After numerous trials and errors, they came up with a triple cuff configuration, eliminating the preconception that a single cuff must be used. It was a product of a drastic change in the way of thinking: they managed to downsize the device by dividing the roles previously played by a single cuff into three, namely, compressing the wrist, detecting pressure on the wrist being compressed, and accurately measuring pressure on the arteries on the skin surface, and then allocating them to separate cuffs*3.
After testing their invention on men and women of a wide age group, they realized the practical use of blood pressure monitors with the triple cuff configuration, achieving uncompromised measurement accuracy with a 25 mm-wide cuff on its inner side, less than half the width of conventional models.

Wearable Blood Pressure Monitor with a Triple Cuff Configuration
*2 The method for measuring blood pressure long used for automated blood pressure monitors at hospitals/clinics and home-use blood pressure monitors. The cuff (airbag inside the blood pressure monitor) pressurizes the upper arm or wrist to occlude the arteries and then gradually lowers its pressure. The monitor detects small pressure oscillation in the cuff's inner pressure that occurs in the process because of the difference between the pressure the cuff puts on the arteries and the pulsation of blood pressure inside the arteries. Blood pressure is measured by comparing the magnitude of the vibrations and the pressure inside the cuff.
*3 For details, please see the paper on OMRON TECHNICS titled "Arterial Compression Technology for a Watch-type Blood Pressure Monitor."
https://www.omron.co.jp/technology/omrontechnics/2020/20200518-kubo.html
(An English translation will be uploaded later on.)
The Efforts Toward "24/7 Continuous Monitoring" Continue

This wristwatch wearable blood pressure monitor was granted clearance as a medical device from the U.S. Food and Drug Administration (FDA) within the shortest-ever span of two months from application to approval: it usually takes around as many as five months. Encouraged by this unprecedented approval, they were able to get over the hurdles leading up to mass production without a hitch. They then received approval for medical devices in Japan and Europe, where the device is available now.
But the development team isn't standing still, as they have embarked on another challenge of further downsizing and realizing 24/7 continuous monitoring of blood pressure. Meanwhile, clinical research has begun on techniques to predict or lessen the risk of developing cerebrovascular and cardiovascular events by analyzing the correlation between blood pressure and other vital data, such as ECG, sleep, and activity.
The wearable blood pressure monitor undoubtedly marked massive progress for the engineers who are working on innovations for achieving zero events, but at the same time, it is just the first step in their quest of Going for Zero.

The Team of Engineers Who Gave Rise to the Wearable Blood Pressure Monitor (Center in the Front Row: Nishioka, Center in the Back Row: Kubo)
 |
NISHIOKA Takanori |
 |
KUBO Takeshi |

 Virtual Showrooms
Virtual Showrooms

 OMRON Taiyo : Embodying the Corporate Motto and Respect for All
OMRON Taiyo : Embodying the Corporate Motto and Respect for All

 Solving Through Automation: Providing Flexibility and Sustainability with Robotics
Solving Through Automation: Providing Flexibility and Sustainability with Robotics
 Co-Creating World's First Bandsaw Safety Solution with OMRON Technologies
Co-Creating World's First Bandsaw Safety Solution with OMRON Technologies
 Creating a New Service for Reliable Operation of Solar Power Generation Facilities
Creating a New Service for Reliable Operation of Solar Power Generation Facilities







